Unlock Your Study Insights in Real-Time
Apollo™ makes it accessible. Boldly challenge scientific possibility with reliable data to ensure patient safety and efficacy, because time matters. You can access and share study-related information in a secure environment with uncovering a new world of speed and accessibility.
The Ultimate Toxicology Experience
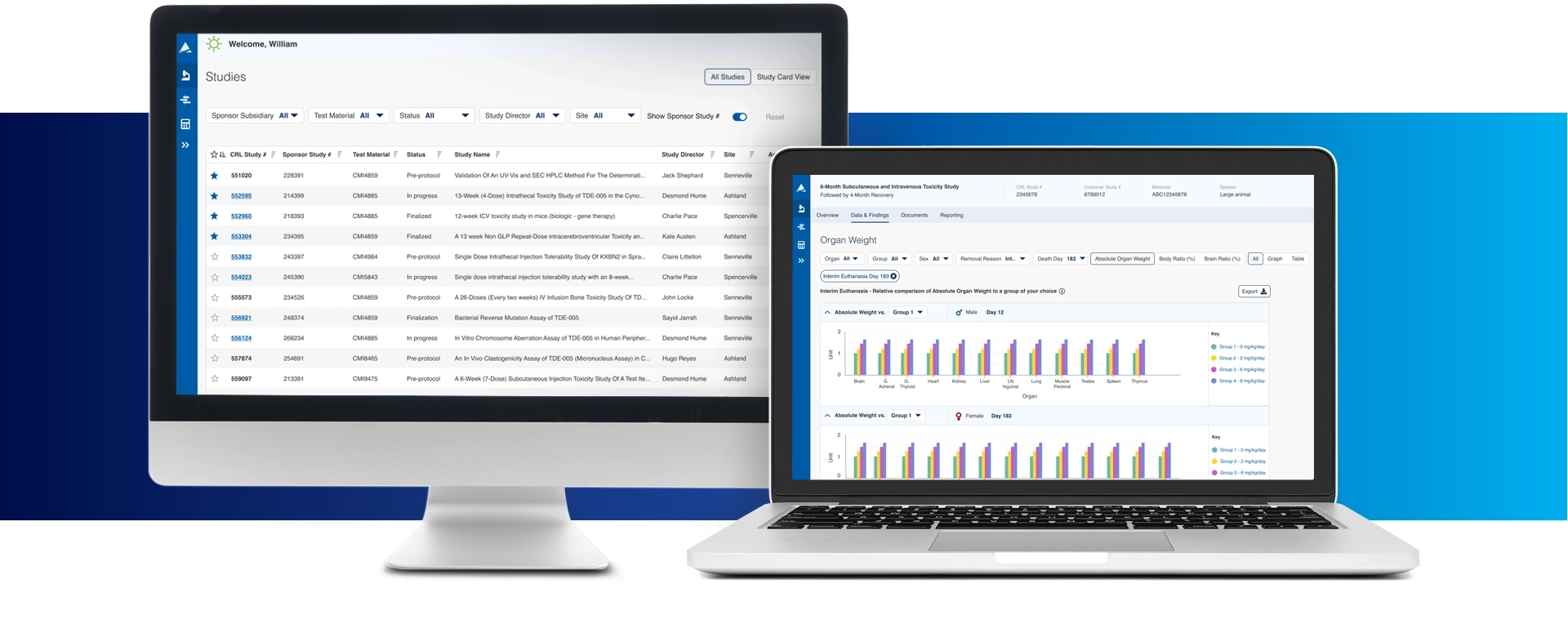
Apollo is an innovative platform that empowers you with real-time access to data visualization, milestones, documents, and program planning tools.
Study Data
Access study data that empowers you with insights and digital features to enhance your decision-making abilities.
Program Milestone
Complete program oversight through study visibility, progress, and deliverables allowing you access where and when you need it at a click of a button.
Document Sharing
Additional document sharing features that are intuitive and seamless for an improved user experience.
Log In to Apollo for Safety Assessment
Since its implementation, Apollo™ has been an instrumental tool in managing our nonclinical studies at Charles River Laboratories. The shared portal allows real-time data to be visualized during the in-life phase of the study through a more secure method of sharing confidential documentation than traditional email.”
WAVE LIFE SCIENCES
Frequently Asked Questions (FAQs) on Apollo™ for Safety Assessment
- How can I login to Apollo as an existing user?
-
Which of my studies can I see in Apollo?
If you are a current Charles River customer, your studies are live on Apollo. The scope of studies in Apollo currently includes studies awarded in the last 18 months, in progress or that have been finalized in the last 90 days. Studies are typically assigned shortly after award to a Study Owner for self-management of study access.
-
How do I access Apollo as a new customer?
You will gain access to Apollo once your studies are awarded with Charles River. Shortly after study award, a primary study contact will be assigned and you will receive access to your account. In the meantime, contact us with your questions.
With continuous agile development of new features and datasets, Apollo is designed to meet your evolving needs.
